How hot air balloons fly: because the hot air inside the balloon is less dense than the air outside of the balloon. The principle is really very simple; hot air rises and cold air sinks.
How Hot Air Balloons Fly
Hot air balloons fly because the hot air inside the balloon is less dense than the air outside of the balloon. The principle is really very simple, hot air rises and cold air sinks.
To make the air inside the balloon hotter, a balloon pilot can turn on the propane burner. When the pilot wants the air inside the balloon to be cooler they can simply allow it cool naturally (everything hot cools down if the heat is removed) or if they want to speed up the cooling process they can let some hot air out of the balloon using a vent panel in the top of the balloon.
Ideal Gas Law
The ideal gas law is a mathematical relationship between pressure,volume and temperature of a gas. For a given quantity of gas the pressure, P, multiplied by the volume, V, divided by the temperature, T, remains a constant.
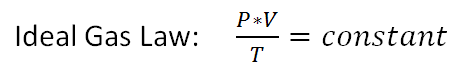
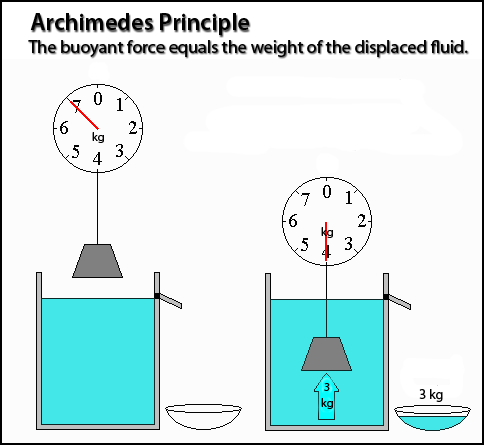
Archimedes Principle
Any object, wholly or partly immersed in a fluid, is buoyed up by a force equal to the weight of the fluid displaced by the object. Most people think of a fluid as being a liquid, however in science a fluid is defined as either a gas or a liquid.
Calculating Lift
Lift = (Air density outside of the balloon – Air density inside balloon) * Volume of the balloon
The lift of a balloon can be calculated by knowing the temperature of the air inside and outside of the balloon and the volume of the balloon. The lift or buoyant force equals the difference in weight of the heated air inside the balloon and the weight of the same volume of air at the surrounding ambient temperature. The table gives the density of air at sea level for various temperatures.
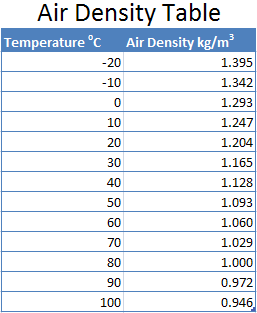
Now let us look at two examples of a typical 4 person balloon which has a volume of 2,200 m3.
If the ambient temperature was 10oC and the air inside the balloon was heated to 100oC the balloon would have a lift of:
2,200 * (1.247 – 0.946) kg = 2,200 * 0.301 kg = 662.2 kg.
If the same balloon was heated to 90oC the balloon would have a lift of:
2,200 * (1.247 – 0.972)kg = 2,200 * 0.275 kg = 600.5 kg
You can see that the hotter the air is inside the balloon the more lift the balloon will generate. In this example by increasing the ttemperature of the air inside the balloon by 10oC from 90oC to 100oC the balloon can lift an additional 61.7kg.
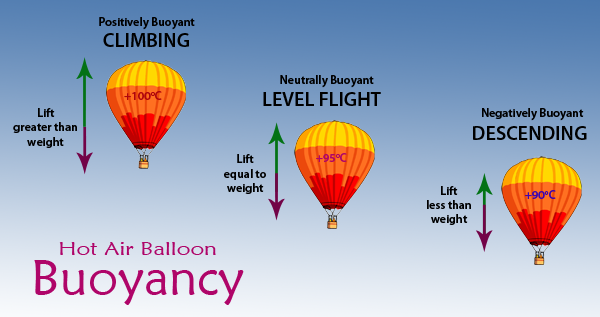
Buoyancy
When we talk about buoyancy in a balloon we usually refer to the balloon as being either positively, neutrally or negatively buoyant. These terms refer to whether the balloon has more lift than weight (positively buoyant), the same lift as weight (neutrally buoyant), or less lift than weight (negatively buoyant).
A balloon that is positively buoyant will climb, a balloon that is negatively buoyant will descend and a neutrally buoyant balloon will remain at the same level. If our example 2,200 m3 sport balloon was loaded so that it weighed 634kg it would maintain level flight if the envelope temperature was 95oC, it would climb if the envelope temperature was hotter than that, say 100oC and it would descend if the envelope temperature was less than 95oC.
Technical data content credited to Mr Steve Griffin
