This article describes the procedure to calculate air density given the temperature, dewpoint and pressure or temperature, pressure and relative humidity. Included on the page are online calculators.
The density of dry air can be calculated using the ideal gas law, expressed as a function of temperature and pressure:

As an example let us calculate air density of dry air at sea level in international standard atmosphere conditions of pressure (1013.25 hPa or 101325Pa) and temperature (15oC).
Remember that to convert oC to oK you need to add 273.15.
Density = 101325 / (287.05 * (15 + 273.15)) = 1.225 kg/m3
You can use the calculator below to work out other examples.
[calculator_pras type=”dry_air_density”] [clickToTweet tweet=”Calculate air density with this online calculator from Brisbane Hot Air Ballooning.” quote=”Has our Air Density calculator helped you? Let others know!”]The Gas Constant (R)
The gas constant is measure of the weight of the number of molecules of a gas. In the atmosphere around us we have air which is about 78% Nitrogen (N2) , about 21% Oxygen (O2) and about 1% other gases. Nitrogen has a molecular weight of 14 so a N2 molecule has a molecular weight of 28. Oxygen has a molecular weight of 16 so an O2 molecule has a molecular weight of 32. Given the mixture of gases found in air the average molecular weight of air is around 29.
Water is made up of two hydrogen and one oxygen atoms (H2O). Hydrogen is the lightest element and has a molecular weight of one. So a water molecule has a molecular weight of 18 (2 * 1 + 1 * 16 = 18). Water is a very light molecule and much lighter than the average weight of the molecules found in air.
Moist Air
In the real world the air always contains some moisture. The addition of water vapour to a mass of air makes it less dense. Whilst this may appear a bit odd at first this occurs because the molecular mass of water (18) is less than the molecular mass of air (29).
The density of humid air can be calculated as the sum of the densities of the two gases, dry air and water vapour in proportion with their partial pressures.
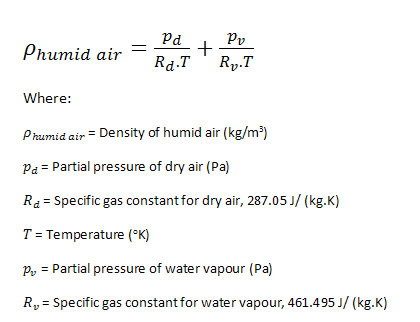 .
.
Calculating Water Vapour Pressure
The amount of water vapour that a parcel of air can hold varies with temperature. The warmer the air the more moisture that it can hold. Air is said to be saturated when the temperature and dew point of the parcel are equal.
There are a number of algorithms available for calculating vapour pressure but we shall use a polynomial developed by Herman Wobus.

The following tools allow you to calculate the density of moist air by knowing either the temperature, pressure and relative humidity OR temperature, dew point and pressure.
The density of moist air is calculated as the sum of the density of the dry air component of the mixture plus the density of the saturated component of the mixture. In the first calculator, the vapour pressure of water vapour in saturated air at the nominated temperature is calculated and multiplied by the relative humidity to give the actual water vapour pressure. The water vapour pressure is then subtracted from the total pressure to give the pressure of the dry component of the parcel. Densities of the two components are then calculated and summed to give the final answer.
[clickToTweet tweet=”Calculate moist air density with online calculators from Brisbane Hot Air Ballooning.” quote=”If you’ve found our Moist Air Density calculators useful, let others know!”] Balloon Pilot and Crew Education – How to Calculate Air Density – Technical data content credited to Mr Steve Griffin
